GCSE Science Subject Content
Describe how heating a system will change the energy stored within the system and raise its temperature or produce changes of state.
Describe how, when substances melt, freeze, evaporate, condense or sublimate, mass is conserved but that these physical changes differ from chemical changes because the material recovers its original properties if the change is reversed.
Define the term specific heat capacity and distinguish between it and the term specific latent heat.
Describe and calculate the changes in energy involved when a system is changed by heating (in terms of temperature change and specific heat capacity).
Details of the Science Content
Energy is stored inside a system by the particles (atoms and molecules) that make up the system. This is called internal energy.
The amount of energy needed to change state from solid to liquid and from liquid to gas depends on the strength of the forces between the particles of the substance. The nature of the particles involved depends on the type of bonding and the structure of the substance. The stronger the forces between the particles the higher the melting point and boiling point of the substance.
The increase in temperature of a system depends on the mass of the substance heated, the type of material and the energy input. The following equation, given on the Physics equations sheet, applies:
change in thermal energy = mass × specific heat capacity × temperature change
∆ E = m c ∆ θ
change in thermal energy, ∆E, in joules, J mass, m, in kilograms, kg
specific heat capacity, c, in joules per kilogram per degree Celsius, J/kg °C
temperature change, ∆θ, in degrees Celsius, °C
The specific heat capacity of a substance is the amount of energy required to raise the temperature of one kilogram of the substance by one degree Celsius.
The energy needed for a substance to change state is called latent heat. When a change of state occurs, the energy supplied changes the energy stored (internal energy) but not the temperature.
The specific latent heat of a substance is the amount of energy required to change the state of one kilogram of the substance with no change in temperature.
The following equation, given on the Physics equations sheet, applies:
energy f or a change o f state = mass × specific latent heat
E = m L energy, E, in joules ,
J mass, m, in kilograms, kg
specific latent heat, L, in joules per kilogram, J/kg
Specific latent heat of fusion – change of state from solid to liquid.
Specific latent heat of vaporisation – change of state from liquid to vapour.
Scientific, Practical and Mathematical Skills
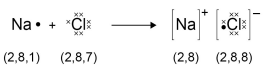
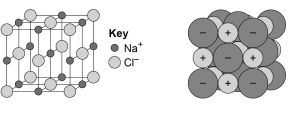
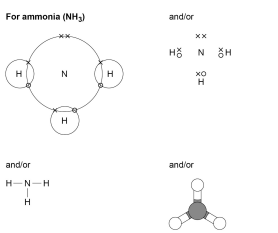
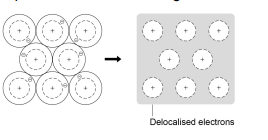
This topic links with Structure and bonding.
(page 98)
WS 3.3
Carry out and represent mathematical and statistical analysis.
WS 3.5,
MS 4a
Interpret heating and cooling graphs that include changes of state.
WS 4.3, 4.5,
MS 1a,3c, 3d
Apply this equation, which is given on the Physics equations sheet, to calculate energy changes when
a material is heated or cooled.
WS 3.3
Carry out and represent mathematical and statistical analysis.